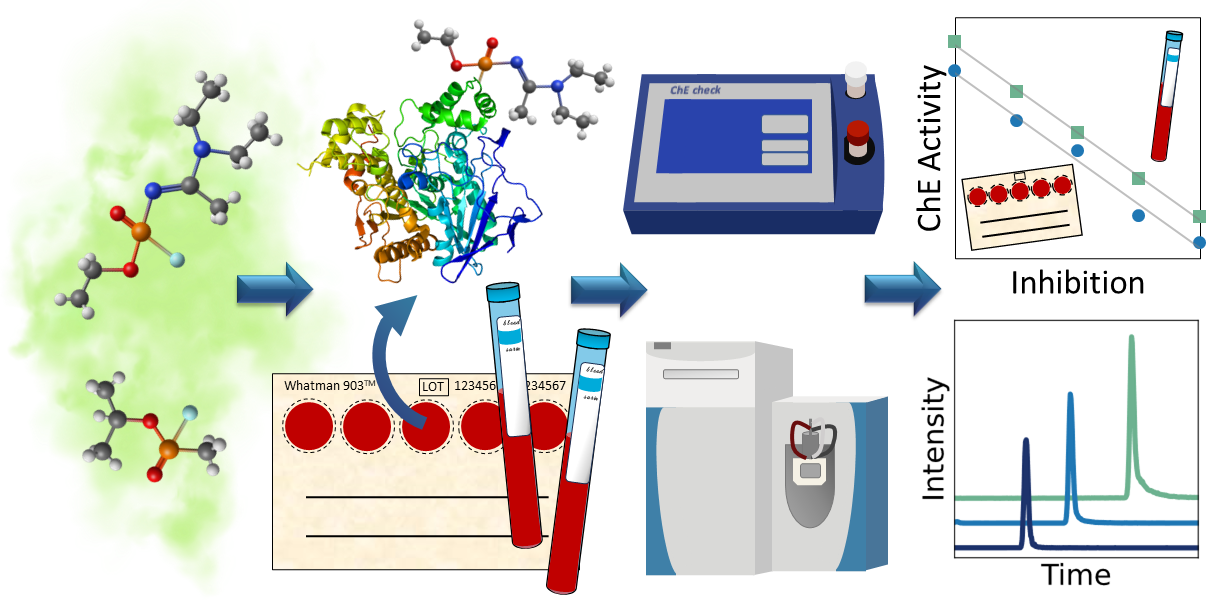
The continuing threats of military conflicts and terrorism may involve the misuse of chemical weapons. Therefore, we studied using dried blood spots for verification of exposure to chemical weapons. We used portable detection and a variety of laboratory analyses for detecting sarin and Novichok nerve agent biomarkers.
One of the remaining challenges in chemical impurity profiling concerns the stability of the actual sample during sampling, transportation, storage and analysis. In this study we were able to identify stable biomarkers in the dried blood and measure the cholinesterase activity using a mobile test kit months after exposure. Both LC-MS/MS and GC–MS/MS were used for unambiguous verification.
"One of the remaining challenges in chemical impurity profiling concerns the stability of the sample."
Read more about this research in Forensic Chemistry